Editors Note: This is a fairly technical report on a change that has occurred to the technology we commonly refer to as “waterproof/breathable” for outdoor apparel. The short version is the chemical composition changed from C8 to C6 while you weren’t looking. What follows is the reason why, and what it means going forward.
In 2005 an EPA Science Advisory Board report found PFOA to be a likely human carcinogen.
One year later Stephen L. Johnson, EPA Administrator obtained commitments from eight major producers of perfluorooctanoatic acid (PFOA), to phase out its manufacture. The EPA’s aim was to eliminate PFOA from “facility emissions and product content” and other precursors or similar chemicals that could break down or function like PFOA. Known as the 2010/15 Stewardship Program, the Agency’s goal targeted 2010 with a 95% worldwide reduction of PFOA and to eliminate it by 2015’s end.
Problems with PFOAs
EPA animal studies have shown relationships between PFOA and “developmental and other adverse effects in laboratory animals.”
Additionally, The Natural Resources Defense Council (NRDC) lists the following potential side effects from PFOA exposure:
- Thyroid disease
- Poor birth outcomes
- Attention-Deficit Hyperactivity Disorder (ADHD)
- Heart disease/higher cholesterol
A host of other problems have been linked to infants exposed, post and prenatally, to PFOA, including low IQ and learning disabilities.
Worse, it appears low levels of PFOA have been found throughout the environment and in 98% of the general human population. It is persistent over time in both humans and the environment. The operative word here is persistent — it resists safely going away. Its stick-to-it-iveness and endemic cast makes the associated problems formidable. Yet its tenacious stability is the reason why PFOA and its family of like chemicals are so extremely useful.
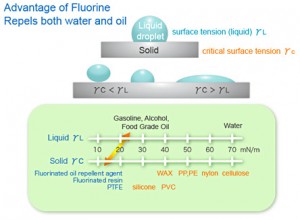
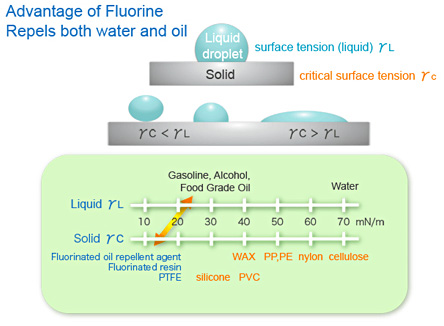
The secret to the functionality of this class of fluorocarbon compounds lies in the strong chemical bond between the fluorine atom (the fluorine in fluoro), the carbon atom and fluorine’s unique character. The resulting fluoro-carbon bond is persistently stable in many environments.
Within this group, also referred to as perfluorocarbons (PFC), a class of industrial chemicals was created. One of these compounds, PFOA, is also known by its contraction C8, is used as a building block in the production of fluoropolymers. Another widely used PFC is perfluorooctane sulfonate (PFOS), a powerful surfactant whose range of usefulness includes functioning like detergents, emulsifiers, dispersants and foaming agents. Collectively, this group of PFCs includes C5, C6, C6 sulfonate, C7 and homologs (longer chains of the same base unit common to these particular PFCs) C9 through 12.
The Benefits of PFCs
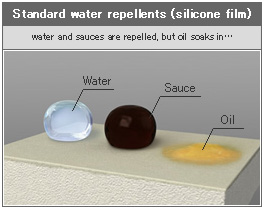
As it turns out, the outdoor recreation industry has been using PFCs for almost as long as they have been around. PFOS is found within the many textiles used in making apparel, tents, sleeping bags and backpacks. PFOS helps keep outdoor fabrics water resistant as durable water repellents (DWR). 3M’s ScotchgardTM was one of the best known PFOS-derived DWRs, bringing stain and water repellency to a wide range of consumer products including furniture, curtains, floor coverings and backpacking gear.
PFOA derivatives were exploited for their low friction, non-stick, heat-resistant properties particularly with the invention of Teflon®. Is there a household in the late ‘60s not taken up by the space-aged miracle of non-stick frying pans? The space age also saw the use of Teflon® in space suits and eventually in other technology spin-offs as in the waterproof laminate membranes Gore-Tex® and eVENT®. For the outdoor retail industry, it is clear PFCs are pretty special, indeed essential to the integrity of many outdoor recreation products.
Balancing Economics with Stewardship
According to the American Chemistry Council (2013), “Globally, FluoroTechnology materials and products specific to the outdoor apparel and equipment industry generate a total of $27.3 billion in economic output.”
Its ubiquity requires the replacement solutions or options to be carefully vetted. Given that it is 2015, will the pact made by the major producers of PFOA reach the EPA’s goal of eliminating them as set out in the 2010/15 Stewardship Program? How will the new products balance performance with environmental compliance?
Jessica Bowman, executive director of the FluoroCouncil stated, “New reporting data published by U.S. EPA in January 2015 show that all Stewardship Program companies are on track to meet the goal of globally phasing out these chemicals by the end of 2015.”
The FluoroCouncil is a global trade association representing the world’s leading FluoroTechnology companies. Founded in 2011, our membership is composed of companies that manufacture fluoroTechnology products: fluoropolymer products, fluorotelomer-based products, fluorosurfactants, and fluoro-surface property modification agents. The members of the FluoroCouncil are Archroma Management LLC, Arkema France, Asahi Glass Co., Ltd., Daikin Industries, Ltd., DuPont Company, and Solvay Specialty Polymers.
For the outdoor industry, it is performance and environment stewardship that drives many of the brand identities and the PFC industry has responded. As part of the voluntary 2010/15 Stewardship Program, the participating companies were to report on their production and emissions, both domestically and by “Non-US Operations.” The yearly reports were to start in 2007 and end in 2015.
Progress so far
Of the eight companies participating in the 2010/15 Stewardship Program, 3M was quiet vocal about getting rid of PFOA, PFOS (not covered under the Stewardship Program) and their precursors early on. Dan Hakes of 3M said, “by late 2001 a substitute had already been made for PFOS,” the mainstay in ScotchGardTM.
3M reported no domestic production of PFOA and their associated precursors by the first report in 2007. By 2012, 3M’s Non-US Operations had ceased production per the EPA’s Stewardship Program as well.
Like all the perfluorocarbon manufacturing companies, DuPont shifted their research towards developing new PFCs with C8 performance minus the drawbacks. Many of these new products have six carbons (C6 or perfluorohexanoic acid (PFHxA)) or less, jointly known as short-chain technology. The short chain advantage lies in:
- Not degrading into PFOA or PFOS
- Does not bio-accumulate
- Has greatly reduced toxicity in all environments
- Is rapidly eliminated from biological systems
DuPont’s Lisa P. Hardy, North America Manager for Teflon® fabric protector, clearly states, “The short chain alternatives now used in Teflon® fabric protector have…no compromise between performance and compliance.”
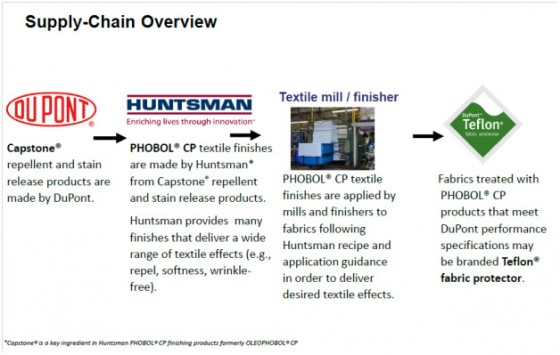
DuPont has 60 tailored solutions marketed under Capstone® for various applications. For textiles, seven Capstone® stain and rain products are converted by Huntsman, a manufacturer of textile finishes, into fabric-specific treatments (Figure 1). For instance, the textile mill would apply Huntman’s PHOBOL CP-S on wool and wool blends. Other products are for cotton or synthetics, with or without stain release. All of the solutions are specific to the end use — not a one size fits all application.
The Swiss-based company Archroma Management LLC created Nuva® N series, consisting of products based on the latest C6 chemistry. However, Archroma also has invested in a fluorine-free technology, Arkophob® FFR, a product competitive with C6 fluorochemicals and alleged to be superior to other flurorine-free DWRs.
Asahi Glass Co., Ltd, has moved its efforts into the C6 realm with AsahiGuard E-SERIES. It is a PFOA-free fluorochemical because they “are recognized as superior to wax or silicone agents because of their superior stability and durability in real-world conditions.”
Since 1968, Daikin Industries, Ltd has been creating UNIDYNE DWRs. Now utilizing C6 backbones in their chemistry, the UNIDYNE product family is PFOA-free. Corporate-wide, Daikin “and its subsidiaries intend to stop manufacturing, using and selling Perfluorooctanoic Acid (PFOA) and C8 telomer-based water and oil repellent products by the end of 2015.”
Additionally, Daikin has five unique product offerings for textiles. They are hybrids using fluorine and silicon, co-developed with Dow Corning Corporation. Marketed under UNIDYNE Multi-Series, the concept is to overcome texture hardening found with certain textile surfaces after being subjected to DWR treatments. The addition of silicon adds suppleness without losing the stain and rain properties of fluorotechnology. Solvay Specialty Polymers’ Fluorolink® PFPE is a product line engineered to solve water and stain repellency on various substrates. In apparel, Fluorolink® 5032 and P56 offer a water-based option or direct application of this perfluoropolyether layer. Its unique structure enables it to attach to synthetic or protein-based textiles while preserving its fluorochemical performance. Solvay emphatically states, it “is not manufactured with, do not containing and do not degradating [sic] to PFOA, PFOS or C8 telomer-like structure.”Is C6 really better than C8?
No less involved, the EPA has worked closely with the companies in using peer-review science to ensure the new compounds do not repeat history with a trove of unforeseen consequences. In addition, the EPA is proposing to apply the Significant New Use Rule (SNUR) on the phased out 2010/15 Stewardship Program chemicals. Under the Toxic Substances Control Act of 1976, SNUR would pre-screen any of the phased out PFCs for any possible new use and after a 90 day review period, deliberate on the extent of its usage.
There is no shortage of non-C8 fluorochemical products for the outdoor industry to choose from post-EPA Stewardship Program. Remarkably, one the salient aspects of the EPA’s Program are how the elimination of PFOA, PFC precursors and homologs was carried out voluntarily. Eight major producers of these invasive, dangerous and very robust fluorochemicals, were able to join the EPA in solving, if not addressing, one of the most dauntingly ubiquitous environmental problems ever faced: all done without the leverage of regulation. There is no question some motivation lies within the “more than $1.2 trillion of global manufacturing output relying upon fluorotechnology” as reported by Bowman in her 29 January 2014 OECD webinar.
A quick and seamless transition to a predominately C6 economy would forego any fiscal disruption of this manufacturing sector. However, some have questioned the efficacy of C6 chemistry as sulfonates like PFOS, with less than eight carbons, may likely bio-accumulate. Be that as it may, the process encourages conversations on mitigating global risk, non-fluorotechnologies or nature-inspired engineering, to all together avoid the overt cost to the planet and its inhabitants.
© 2015
Related Posts
DuPont found liable in Teflon Toxin Trial
SNEWS: DWR is yucking up the planet

3 comments
Thanks for the detailed summary! The bottom line for the user is that so far, the C6 DWR coatings simply aren’t as good as the C8 coatings. Some of the leading companies (e.g., ArcTeryx) moved ahead with the change to C6 2 years ago, others did so last year and the rest are doing it now. I’ve been surprised there hasn’t been more gnashing of teeth about the poorer performance of waterproof-breathable garments…
From my perspective, it is quite clear that the C6 DWR fabrics wet out sooner and the DWR has to be refreshed more regularly. Personally, I wonder about the efficacy of the change; if you have to use a spray-on DWR a few times a season while before it would take several years before you needed to even do it once – isn’t there a lot more chemical going into the environment? Is 10x the amount of C6 vs C8 actually better?
I hope the chemical companies develop a new/improved DWR soon!
Nice work Dostie — thanks for the info! Looks like it’s back to cotton and stainless steel for me…
Didn’t re-read this (first some weeks ago) but wanted to post up “many thanks.” Some time ago I went on a mission, obsessed, to discover the real truth of DWR. A fresh, new beading, DWR is so wonderful…and nearly impossible to re-establish. What’s the chemistry? User comment is the standard useless: “I’ve had great success with…” Some mfg sites Tech FAQs come close, but invariably fizzle before giving the real answer (real goal is informed uncertainty and demand for “specialty washes treatments.”). There are loads of herding-words: “harsh detergents,” “pure soap.” All soaps are detergents; soaps combine with calcium as scum. Drifted away from my project but did identify fluro based as the durable one I wanted (& Revivex hand pump spray as having more fluro and less propane). Before reading you, I’d have gone with PQ as favoring fewer applications, if somewhat more toxic. But I’m happy to be less toxic, too….glad your chemists are plugging away!